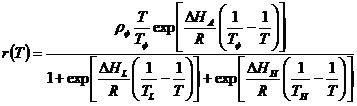|
Nymphs and adults of planthoppers suck sap from plants. Under favorable conditions they multiply very fast. Both brown planthopper(BPH) and white backed planthopper(WBPH) are known for their resistance to commonly used insecticides including the neonicotinoids. Hence crop failures due to severe pest outbreaks are very common in many rice-growing tracts of India. Temperature, relative humidity and prevailing wind direction determine the severity of incidence and spread of BPH. Several simulation models developed in China for BPH are based on occurrence patterns over locations, seasonal temperatures, transplantation time and immigration pattern. Attempts have been made to differentiate healthy and affected plants using spectroradiometry.
Description
The brown planthopper (BPH), Nilaparvata lugens Stal (Hemiptera: Delphacidae) is a major insect pest that causes enormous yield loss in rice, Oryza sativa L., in Asia [1-4]. Due to its short life cycle and high fecundity, population increases quickly reach high density levels, cause severe crop damage due to sucking of sap and oviposition in stem tissues which results in drying up of infested plants in concentric circular patches (typical ‘hopperburn’ symptoms) [5]. Apart from direct plant injury, the insect also acts as a vector for transmitting grassy stunt and ragged stunt virus diseases [6-7]. In recent years, frequent outbreak of BPH in Asian countries is attributed to expansion in rice and development of high levels of insect resistance to major insecticides [8-9]. BPH is monophagous, feeding only on rice (Oriza sativa L.) and wild-rice spp. However, in no-choice experiments, BPH could feed or oviposit on finger millet (Eleusine coracana G.), sugarcane (Saccharum officinarum L.), rice cut grass (Leersia hexandra Sw.), maize (Zea maysL.), Jungle rice (Echinochloa colona L.), nut grass (Cyperus rotundus L.), sorghum (Sorghum vulgare L.) and wheat (Triticum aestivum L.) [10]. N. lugens is distributed in Asia, Australia, and the Pacific Islands. In Asia, it is found in Bangladesh, Brunei, Burma (Myanmar), China, Hong Kong, India, Indonesia, Japan, Cambodia, Korea, Laos, Malaysia, Nepal, Pakistan, the Philippines, Singapore, Sri Lanka, Taiwan, Thailand, and Vietnam. In Australia and the Pacific Islands, it is found on the Caroline Islands, Fiji, Mariana Islands, Papua New Guinea, and Solomon Islands [6]. It is not found in America and Africa. BPH is mainly a pest of irrigated rice, but be found in rainfed environments. However, it is rare in upland rice [6]. The life cycle of N. lugens comprises two immature stages i.e., egg and nymphal stages prior to the adult stage. Each generation requires about 30 days for completion and is mostly dependent on prevailing temperatures. The insect can occur in 6 to 8 generations in a year. Adult: The adult stage is dimorphic i.e., two morphotypes, one with long forewings (macropterous) and the other with short wings (brachypterous) [11]. In females, crowding promotes frequency of macropterous forms. In males, a moderate nymphal density promotes brachypterous forms [12]. The short winged brachypterous adults exhibit high fecundity, while the long winged macropterous adults can migrate over long distances. Adult longevity varies from 10-20 days depending on temperature. Gravid female
Egg Females lay eggs by penetrating plant tissue with the ovipositor where eggs are laid in groups [13], mainly in leaf sheaths, but also in the leaf blade. Prevailing temperatures shortly after adult emergence influence oviposition of macropterous females [14]. Short winged adult morphs lay more eggs than macropterous forms [15]. Newly laid eggs are whitish and turn darker before hatching. About to hatch eggs are identified by the appearance of two red eye spots of the nymph inside. In most cases, eggs are thrust in a straight line, generally on the lower portion of the host plant along the mid-region of the leaf sheath, though sometimes eggs are laid in clusters of 4-10 in longitudinal rows within the leaf midribs [16]. Egg period varies from 7 to 11 days depending on temperatures in the optimum temperature range of 20-30°C.
Nymph
Nymphs have a similar appearance to adults but are smaller, have different coloration, and are devoid of functional wings. Wing buds appear during the fifth instar. The nymphal stage passes through 5 instars and takes 10 to 15 days in the temperature range of 20-30°C to complete development. Kisimoto [17] noted that in N. lugens, nymphal period is shorter and fairly constant for the brachypterous form in both sexes, and even at high densities. However, nymphal duration in the macropterous forms is lengthened by greater density.
N. lugens is an economically important pest that feeds directly on rice plants and also acts as a vector of viruses of rice, resulting in significant damage and yield losses [16]. The most severe outbreak of the BPH in India occurred in Kerala state towards the end of the rainy season in 1973 extending to early part of 1974 [18-19]. Hopperburn symptom often developed in patches and sometimes covered whole fields. Sometimes the damage was so great that growers abandoned the crop [20]. The loss in grain yield ranged from 10% in moderately affected fields to 70% in those severely affected [21]. Chatterjee [22] reported the shift from being a minor pest to a major pest status on rice in two northern districts of West Bengal. Outbreak extended to other districts in West Bengal in 1975, and in several cases the crop was destroyed [23]. Chelliah and Subramanian [24] referred to the cyclical appearance of BPH epidemics once every few years in Tamilnadu that lead to extensive damage. BPH assumed pest status in as many as 10 states in India, including Uttar Pradesh, Bihar, Haryana, and Punjab [25-27]. Globally, N. lugens outbreaks have been reported from South-east Asia in the 1980s with densities as high as 1,000-2,000 per hill [28]. Excessive use of urea as a nitrogenous fertilizer is one of the main causes of outbreaks as it increases the fecundity of N. lugens. The injuries caused by N. lugens to rice plants include a decrease in leaf area, photosynthetic rate, plant height, leaf and stem nitrogen concentration, chlorophyll content and dry weight. Heavy injury usually results in wilting, stunting, and finally death of the plant. This type of damage is called ‘hopper burn’. N. lugens may also transmit the grassy stunt disease which can further reduce yield. The series of slits produced by females when depositing their eggs may also contribute to plant dieback [29].
Life cycles and the population growth of N. lugens are associated with temperature [30-33]. High temperature (above 34°C) and low temperature (below 20°C) have been shown to significantly reduce survival and fecundity [33-34]. The rate of insect development is affected by the temperature to which insects are exposed [35]. Ttemperature conditions during the nymphal stage also affect adult longevity, oviposition rate, and preoviposition period in macropterous females. Temperature can have a significant and quick impact on distributions and abundance because the main eco-physiological trait of insects (e.g. life cycle duration, mobility, reproduction), are all sensitive to the thermal environment. Direct effects could involve impacts on insect development and survival, changes in host defence physiologies, while indirect effects include changes in natural enemy and competitor abundance [36]. Temperature is a key influencing factor on development of BPH under field conditions. To model the development, it is important to estimate lower and upper parameter thresholds for initiation and cessation of development, respectively. This is best done under constant temperatures under laboratory conditions. Complete development of individual rice leaf folder insects reared under a range of temperature conditions (15 to 32°C) was assessed keeping ecologically relevant relative humidity and photoperiod conditions constant across temperatures. The instar development durations were converted to developmental rates and subjected to linear and non-linear regression techniques.
Linear models Two models were evaluated to estimate the linear relationship between ecologically relevant temperatures and the rate of development of the pest. The first was the thermal summation model [35] which is given by the expression,
Where, r is the rate of development (=1/ Development time (D) in days), T is ambient temperature (oC); intercept (a) and slope (b) are the model parameters. Thermal constant, k (= 1/b), is the number of degree-days (DDs) or heat units above the threshold needed for completion of an instar. Lower temperature threshold (Tmin) was determined as the x-intercept (= - a/b) which is the estimated lower temperature at which the rate of development is either zero or no measurable development occurs. The second linear model by Ikemoto and Takai [37] is given as,
Where, DT is the product of the duration of development, D (days), and temperature, T(oC), k is thermal constant and Tmin is the lower developmental threshold.
Nonlinear models Two empirical nonlinear models were fitted to the instar specific developmental rate data to estimate the optimum temperature threshold (Topt) and upper temperature threshold (Tmax). Topt is the threshold temperature at which developmental rate is maximal, while Tmax is the lethal threshold at which development ceases. Lactin-2 model [38], Briere-1 model [39] was applied to assess the nonlinear relationship. In addition, a thermodynamic model referred to as the Sharpe-Schoolfield-Ikemoto model (SSI model) was used to estimate intrinsic optimum temperature for the pest [40]. All the nonlinear models described the relationship between developmental rate (1/D) and temperature (T). The Lactin-2 model [38] is given by the expression,
where, D is the mean development duration in days, ρ is the composite value for critical enzyme-catalyzed biochemical reactions as T increases to Topt, Δ is the difference between Topt and Tmax when thermal breakdown becomes the overriding influence and λ is a fitted coefficient that forces the nonlinear curve to intersect the x-axis and allows the estimation of lower developmental threshold. The Briere-1 model [39] is given by the expression,
where, r is the developmental rate as a function of temperature (T), and ‘a’ is an empirical constant.
A thermodynamic model referred to as the Sharpe-Schoolfield-Ikemoto model (SSI model) was used to estimate intrinsic optimum temperature for the pest [40]. All the nonlinear models described the relationship between developmental rate (1/D) and temperature (T). The nonlinear thermodynamic SSI model [40] is given by the expression:
where, developmental rate (r) is a function of temperature, T (in absolute temperature, K) (273.15K = 0°C), R is the gas constant (1.987 cal/deg/mol), Δ HA is the enthalpy of activation of the reaction that is catalyzed by the enzyme (cal/mol), ΔHL is the change in enthalpy associated with low-temperature inactivation of the enzyme (cal/mol), ΔHH is the change in enthalpy associated with high-temperature inactivation of the enzyme (cal/mol), TL is the temperature at which the enzyme is half active and half low-temperature inactive (K), TH s the temperature at which the enzyme is half active and half high-temperature inactive (K), TΦ is the intrinsic optimum temperature at which the probability of enzyme being in the active state is maximal (K), and ρΦ is the mean development rate at the intrinsic optimum temperature (TΦ ) assuming no enzyme inactivation (day-1).
Used to compare the survival of BPH across different temperatures.
|